Abstract
Background: Minimal/measurable residual disease (MRD) post initial therapy is prognostic of long term outcomes in patients (pts) with newly diagnosed MM (NDMM), but has not been used to modify therapy. We hypothesized that the combination of daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd) would be safe and highly active in pts with NDMM. In addition, we employed MRD by next generation sequencing (NGS) to inform the use and duration of Dara-KRd post-autologous transplant (AHCT) and treatment cessation in pts with confirmed MRD negativity.
Methods: Eligible pts had NDMM requiring treatment, CrCl ≥40 ml/min, adequate liver and heart function, ECOG performance status 0-2 with no age limit. There was a planned enrichment for pts with high-risk cytogenetic abnormalities (HRCA). Treatment cycles consisted of daratumumab 16 mg/kg IV days 1,8,15,22 (with typical reduction in frequency with subsequent cycles), carfilzomib 56 mg/m 2 IV days 1,8,15, lenalidomide 25 mg PO days 1-21 and dexamethasone 40 mg PO/IV days 1,8,15,22 repeated every 28 days. Pts received 4 cycles of Dara-KRd as induction, AHCT, and received 0, 4 or 8 cycles of Dara-KRd consolidation, according to MRD status. MRD was evaluated by NGS (ClonoSEQ®) in all pts at end of induction, post-AHCT, and during each 4-cycle block of Dara-KRd consolidation. Primary endpoint was achievement of MRD negativity (<10 -5 as defined by IMWG) in the intent-to-treat population. Other endpoints included MRD <10 -6 and complete response (CR) by IMWG criteria. Pts received therapy until achievement of two consecutive MRD <10 -5 (confirmed MRD-negativity, i.e., post-induction and post-AHCT or post-AHCT and during consolidation). Confirmed MRD-negative pts entered treatment-free observation and MRD surveillance ("MRD-SURE" phase) with surveillance for MRD resurgence 6 months after treatment cessation and yearly thereafter. Pts completing consolidation without confirmed MRD-negativity received lenalidomide maintenance.
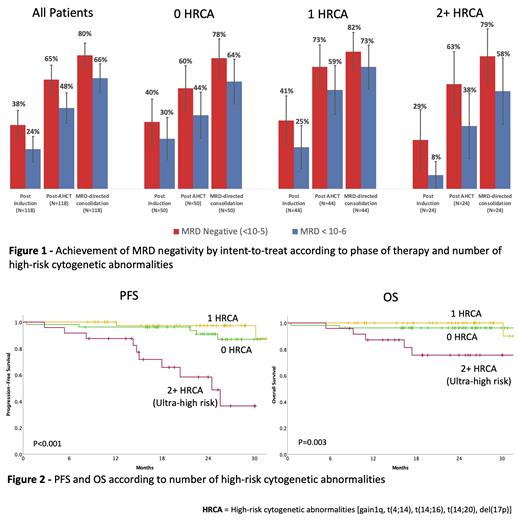
Results: The study accrued 123 participants between 03/2018 and 09/2020. Fifty-three patients (43%) had no HRCA, 46 (37%) had 1 and 24 (20%) had 2+ HRCA [gain 1q, t(4;14), t(14;16), t(14;20) or del(17p)]. Median age was 60 y (36-79) and 20% were 70 or older. Twenty-three percent of pts were non-white, 20% had ECOG 2, 21% had high LDH, and 20% R-ISS3. Disease was trackable by NGS-MRD in 118 (95.9%) of pts. Median follow up is 25.1 mo. Four pts remain on protocol treatment, 20 transitioned to lenalidomide maintenance and 84 (71.2%) have reached confirmed MRD negativity and entered MRD-SURE. For those patients, median follow up post treatment cessation is 14.2 mo. Most common severe adverse events were pneumonia (N=8), and venous thromboembolism (N=3) and 3 patients died during treatment. Overall, 80% of pts have achieved MRD negativity and 66 % MRD < 10 -6. Depth of response improved with each phase of therapy and became similar in patients with 0, 1 or 2+ HR abnormalities as assessed post-AHCT and with MRD-guided consolidation (Figure 1). A similar proportion of patients with 0, 1 and 2+ HRCA reached MRD negativity (78. % vs. 82% vs 79 %) and MRD<10 -6 (64% vs. 73% vs. 58%). Response ≥CR was obtained in 86% of pts. Two-year progression-free survival (PFS) was 87% (91%, 97%, 58% for patients with 0, 1 and 2+ HRCA respectively) and 2-year overall survival (OS) was 94% (96%, 100%, and 75% for patients with 0, 1 and 2+ HRCA respectively, Figure 2). None of the pts reaching MRD-SURE has died from MM recurrence. Cumulative incidence of MRD resurgence or IMWG progression 12 months after cessation of therapy was 4%, 0% and 27% for patients with 0, 1 or 2+ HR abnormalities respectively.
Conclusion: Monoclonal antibody-based quadruplet therapy, AHCT and MRD response-adapted consolidation therapy leads to the highest rate of MRD negativity reported in NDMM. Near all patients with 0 or 1 HRCA and confirmed MRD negativity remain free of IMWG progression or MRD resurgence despite cessation of treatment. While most patients with ultra-high risk MM reach deep responses with this approach, novel consolidative strategies are needed. For most patients with NDMM, this strategy creates the opportunity of MRD surveillance as an experimental alternative to the burden of indefinite maintenance.
Costa: Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Speakers Bureau. Chhabra: GSK: Honoraria. Dholaria: Janssen: Research Funding; Jazz: Speakers Bureau; MEI: Research Funding; Takeda: Research Funding; Pfizer: Research Funding; Angiocrine: Research Funding; Poseida: Research Funding; Celgene: Speakers Bureau. Silbermann: Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Giri: CareVive: Honoraria, Research Funding; PackHealth: Research Funding. Hari: GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau.
Carfilzomib for newly diagnosed multiple myeloma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal